Alanis Therapeutics


Developing BOLD New Antibody
Treatments for
Cancer and Autoimmune Diseases
Alanis Therapeutics, a spin out of the San Diego antibody engineering company AvantGen, is harnessing the potential of antibody-based therapeutics to target novel pathways and develop new agents to treat cancer and autoimmunity. Our lead product, ATI-D1, is an antibody that inhibits notch1 signaling between osteoblasts and leukemic stem cells-a key oncogenic driver of tumor progression in MDS/AML.

About us

In a collaboration with investigators at Columbia University, up to 70% of MDS/AML patients demonstrated activated beta-catenin/Jagged-1 (JAG1) signaling in osteoblasts that was shown to increase with disease severity and correlated with MDS to AML transformation. In preclinical studies, Alanis’ anti-JAG1 antibody (ATI-D1), capable of inhibiting notch signaling, rescued anemia, thrombocytopenia, neutrophilia and lymphocytopenia, restored LT-HSCs (the leukemia initiating population), relieved myeloid differentiation block and eliminated blasts.
Alanis is now developing the antagonist anti-JAG1 antibody for
first-in-man clinical testing in MDS and AML

MDS and AML PLATFORM

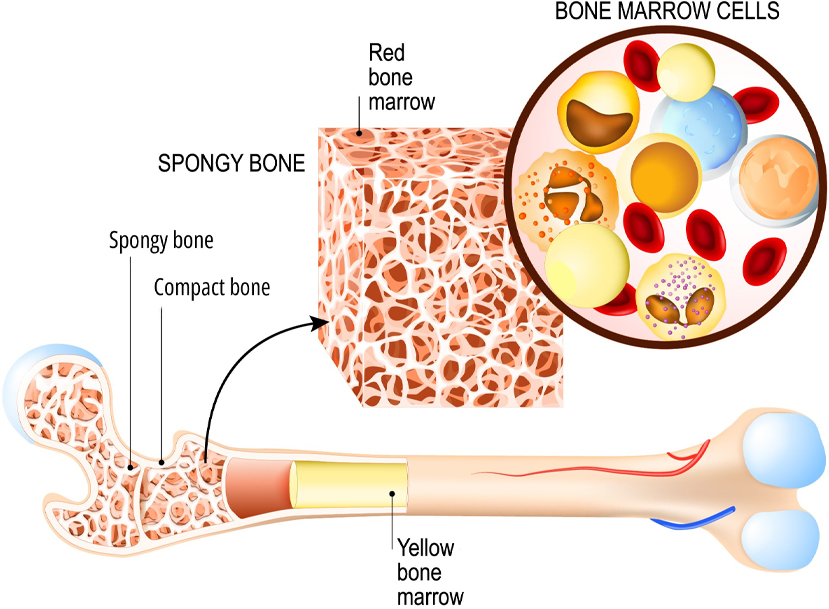
These debilitating hematopoietic disorders lie on a malignant spectrum arising from unstable genetic and molecular changes in hematopoietic stem cells. Disease progression is characterized by an overproduction of immature blood cells leading to anemia, risk of infection and death.
The incidence of MDS is 4.9 per 100,000/year overall and increases to >70 per 100,000 in the elderly. With a prevalence of >70,000, 33% of patients progress to AML. The incidence of AML is ~ 4.2 per 100,000 population and the prevalence is ~20,000.
Standard of care high dose chemotherapy and hematopoietic stem cell (HSC) allogeneic transplant has remained unchanged in the last 3-4 decades. New therapies targeting FLT3 or BCL-2 in combination with chemotherapy have added to the overall toxicity of treatment to improve the rates of complete remission but not the rate of relapse. The relapse rate for high risk AML is still over 60% leading to an overall median disease-free survival of <1 year (range 4-11 months). In patients ≥60 years, the 5 year survival rates drop to 7.4%.
Consequently, new therapeutic hypotheses targeting the “source” of the disease are badly needed to improve treatment outcomes. Increasing evidence suggests that inhibition of the oncogenic or tumor-promoting signals from the bone marrow stroma might overcome mutation dependence and clonal resistance to better control disease and improve clinical outcomes.
Technology/ Science

Jagged-1 expression on osteoblasts triggers Notch 1 signaling in hematopoietic stem cells that lead to AML.
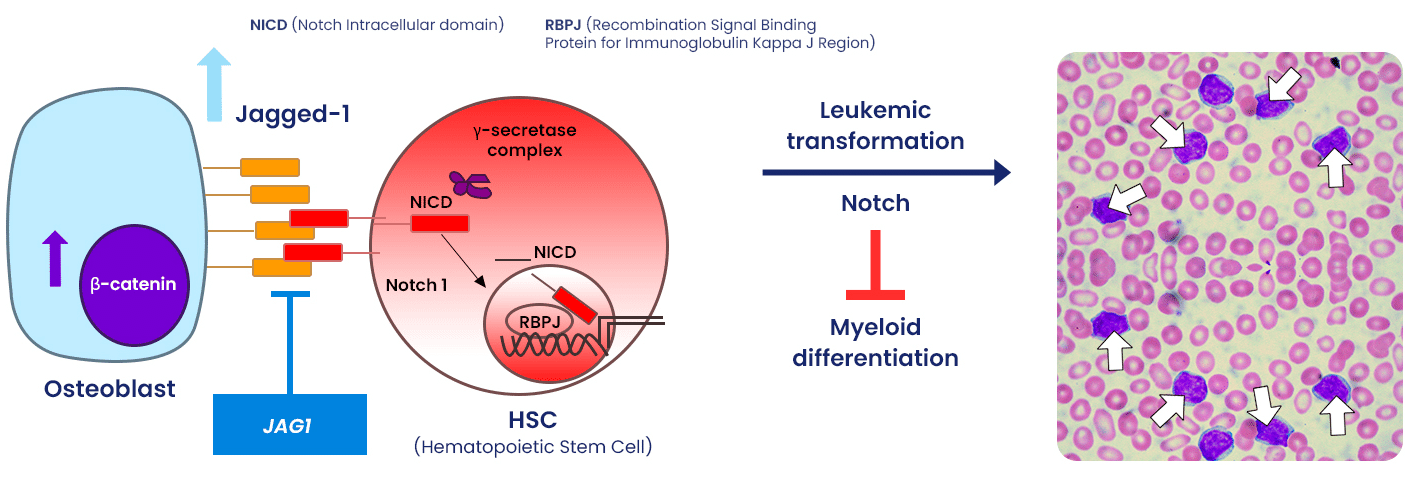
- Lead candidate is an antibody which antagonizes Jagged-1 (JAG1) ligands on osteoblasts in stroma
- Jagged-1 expression on osteoblasts triggers Notch 1 signaling in hematopoietic stem cells that leads to AML
- Mouse model recapitulates pro-MDS/AML environment observed in human patients
- Blocking Jagged 1-Notch 1 signaling prevents/treats MDS/AML:
- Prevents leukemogenesis/leukemia
- Induces apoptosis of MDS and AML cells
- Significantly prolongs survival
- Reverses anemia, neutrophilia & lymphocytopenia
- Attenuates blast infiltration in blood
- The pathway is active in up to 70% of AML and MDS patients across all WHO categories
Increasing evidence points to MDS/AML as niche-dependent genetic malignancies for which stomal cells may be a more stable target for therapy than leukemic clones
Inhibition of oncogenic or tumor-promoting signals from the stroma might be a more effective means of controlling disease and managing relapse in overcoming mutation dependence and clonal resistance
Data show constitutive activation of beta-catenin/Jagged-1 signaling in osteoblasts:
- Is activated in up to 70% of MDS/AML patients where
- Activation levels increase with disease severity and progression that
- Correlate with MDS to AML transformation and
del(5q)-associated myeloid malignancies and - Leads to MDS rapidly progressing to AML in mice
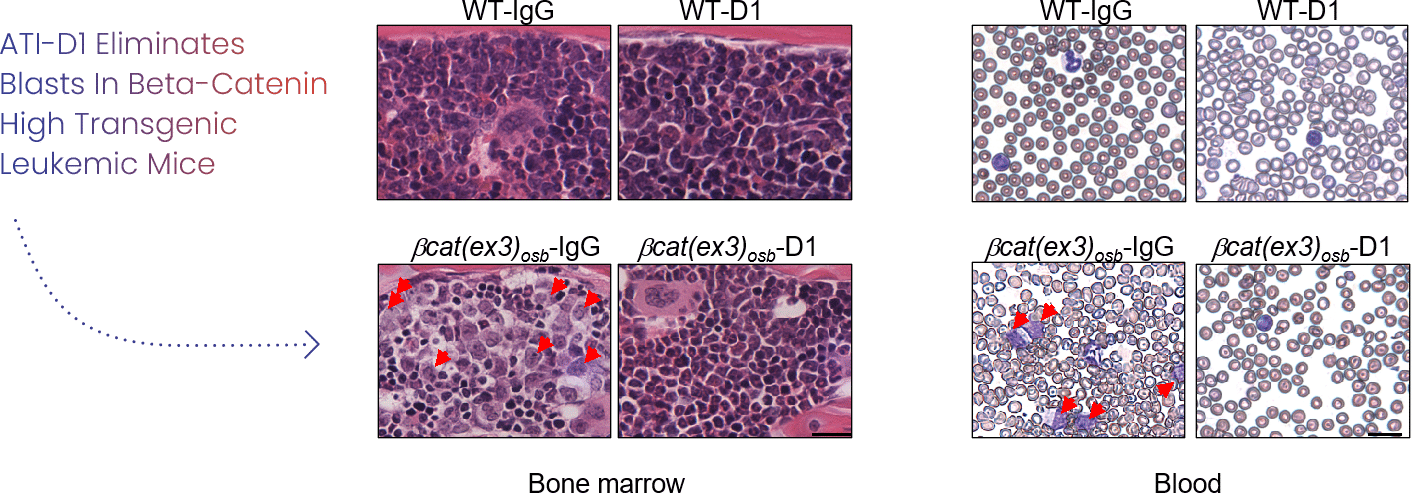
Anti-JAG1 antibodies, capable of inhibiting Notch1, signaling rescues anemia, neutropenia and thrombocytopenia and lymphocytopenia; restored LT-HSCs; relieved myeloid differentiation block and eliminated blasts in mice with beta-catenin active osteoblastic leukemia
In contrast, chemotherapy exacerbated anemia without decreasing blasts
Anti-JAG1 treatment of patient derived samples with activated osteoblastic beta-catenin inhibited MDS/AML cell growth and survival also promoting myeloid and erythroid differentiation irrespective of disease category
Targeting Notch1 signaling through Jagged-1 may therefore provide a broad therapeutic approach effective in a third of MDS and AML patients with cytogenetically distinct types of disease
The therapeutic potential of targeting the bone marrow stromal niche in hematological malignancies can be a treatment strategy that may prevent relapse and overcome standard of care resistance and toxicity
ADDITIONAL PLATFORMS


Alanis has an option to develop additional antibodies created by AvantGen, such as bi- or tri-specific antibodies that engage NK cells for the treatment of cancer or autoimmune diseases (Liao et al., 2024). The platform also includes immunoglobulins directed against molecules associated with disease pathology which can be incorporated in bispecific form to enhance the specificity and therapeutic index for immune targeting.

Leadership


Robert Goodenow, Ph.D. CEO
Dr. Goodenow has held C-suite and BD positions as President of HUYABIO, Chief Business Officer of Syndax Pharmaceuticals and Vice President Corporate Development of Inovio Pharmaceuticals. He has experience in business development, equity financing, drug development and global product launch. Dr. Goodenow received an A.B. in Biochemistry from UC Berkeley and a Ph.D. in Biophysics from Stanford Medical School.

Danelle James, M.D. M.S. Consulting Medical Officer
Dr. James led the global development of Imbruvica (first BTK inhibitor) from first in human studies to 15 global registrations at Pharmacyclics and Abbvie after the $21 billion acquisition in 2015. At Summit Therapeutics, she developed ivonescimab (first in class VEGF-PD1 bispecific) which led to a 5 billion partnership. Dr. James received her M.D. from Pennsylvania State College of Medicine and her Masters in Advanced Studies of Clinical Research from UC San Diego. On the UCSD faculty, she focused on malignant hematology and clinical-translational research. Dr. James has published over 50 peer review manuscripts.


Catherine Woods, Ph.D. Head of Research
Dr. Woods has more then 30 years experience in Pharma and Biotech. She has served as an Exec Director, Research, at Targeted Molecules; Director of Clinical Research and Sr. Director of Biological Research at Alliance Pharmaceutical Corp. She was a Research Fellow at Merck Research Laboratories. Dr. Woods received a Ph.D., UC-Davis and was a Post-doc at California Institute of Technology.

Elizabeth Song, Ph.D. Head of Operations
Dr.Song has held senior management positions in operations in established, emerging and start-up biotechnology companies including HUYABIO, Gemvax and EGeen International. She also has extensive experience in operational and logistics management in clinical contract research organizations. Dr. Song received a Ph.D. in Genetics from University of California, Berkeley and was a Damon Runyon Walter Winchell Fellow at Scripps Institute in San Diego.


Xiaomin Fan, Ph.D. Chairman, Founder
Dr. Fan is currently the President & CEO of AvantGen, the parent company of Alanis Therapeutics. In this capacity, he played a leading role in establishing and developing AvantGen. He is the inventor of the company’s cutting-edge technology platforms. Prior to his leadership at AvantGen, Dr. Fan accumulated valuable experience in diverse scientific positions at Targeted Molecules Corporation. He holds a Ph.D. in Molecular Biology and Biochemistry from the University of Kansas and was a postdoctoral fellow at the University of Pennsylvania.

Cynthia (Cindy) Collins Director
Cindy is a recognized leader with more than four decades of experience in gene and cell medicines, biopharmaceuticals, and diagnostics. She has served as CEO of Editas Medicine (NASDAQ:EDIT), Human Longevity, GenVec and Sequoia Pharmaceuticals as well as CEO/GM of General Electric Healthcare’s Cell Therapy Business, Lab Businesses and Clarient Diagnostics. She also served as Group Vice President, Cellular Analysis Business of Beckman Coulter.
Cindy also served as President of Clinical Micro Sensors, Inc., a wholly-owned subsidiary of Motorola (now GenMark Diagnostics) and held various executive roles with Baxter Healthcare, including President of Oncology, Vice President of Strategy and Portfolio Management of BioScience, Vice President and General Manager of Cell Therapies, and Vice President of Business Development of Transfusion Therapies. She began her career with Abbott Laboratories in various operating roles.
Cindy received her BS degree in Microbiology from the University of Illinois, Urbana, and her MBA, from The University of Chicago Booth School of Business. She is a member of the board of directors of Certara (NASDAQ:CERT), MaxCyte (NASDAQ:MXCT), Draper Laboratories, Nutcracker Therapeutics and Foundation for mRNA Medicines.


Stavroula Kousteni, Ph.D. Chair Scientific Advisory Board
Dr Kousteni is the Edward P. Evans Professor, Department of Physiology & Cellular Biophysic and Director of the Edward P. Evans Center for Myelodysplastic Syndromes at Columbia University. Her laboratory examines the role of the bone marrow microenvironment (or niche) in hematopoietic stem cell fate and function, in particular during the development of MDS and AML. Their focus is on identifying the subpopulations in the bone marrow microenvironment and the mechanisms through which they interact with malignant cells to regulate disease progression. The ultimate goal is to identity therapies that target oncogenic signals stemming from the bone marrow microenvironment to prevent MDS and AML transformation and overcome targeted or standard of care therapy resistance.
Azra Raza, M.D. Chair Clinical Advisory Board
Dr. Raza is the Chan Soon-Shiong Professor of Medicine and Director of the MDS Center at Columbia University in New York, NY. She started her research in Myelodysplastic Syndromes (MDS) in 1982 and moved to Rush University, Chicago, Illinois in 1992, where she was the Charles Arthur Weaver Professor in Oncology and Director, Division of Myeloid Diseases. The MDS Program, along with a Tissue Repository containing more than 50,000 samples from MDS and acute leukemia patients was successfully relocated to the University of Massachusetts in 2004 and to Columbia University in 2010.
Before moving to New York, Dr. Raza was the Chief of Hematology Oncology and the Gladys Smith Martin Professor of Oncology at the University of Massachusetts in Worcester. She has published the results of her laboratory research and clinical trials in prestigious, peer-reviewed journals such as The New England Journal of Medicine, Nature, Blood, Cancer, Cancer Research, the British Journal of Hematology, Leukemia, and Leukemia Research. Dr. Raza serves on numerous national and international panels as a reviewer, consultant, and advisor and is the recipient of a number of awards.
NEWS

August 28, 2023 AvantGen Forms Therapeutic Subsidiary Alanis Therapeutics Inc.
AvantGen, the San Diego Antibody Engineering Company, announced today the formation of Alanis Therapeutics Incorporated (ATI), a Delaware Corporation. Dr. Xiaomin Fan, Ph.D., Founder and CEO of AvantGen will serve as the Chairman of ATI which will develop AvantGen’s antibody platform for various therapeutic applications. This includes the antagonist antibody to jagged-1, the ligand of notch1 signaling, which has been studied in collaboration with investigators at Columbia University as an oncogenic driver between osteoblasts and leukemic stem cells in MDS and AML. Dr. Fan said: “We’ve reached an important milestone in the Company’s evolution to have products with therapeutic potential to be developed by Alanis. We look forward to moving our lead asset into the clinic in collaboration with our investigators at Columbia University.”
October 5, 2023 Alanis Appoints Robert Goodenow CEO.
AvantGen Inc, the San Diego Antibody Engineering Company, announced today the appointment of Robert Goodenow, Ph.D. as the CEO of Alanis Therapeutics Incorporated (ATI). Dr. Goodenow brings extensive executive experience including product approvals and launch to the development of ATI’s therapeutic antibody platform having served in various senior and C-suite roles for HUYABIO International, Syndax Pharmaceutics, Inovio BioMedical, Aventis Pharma and Baxter Healthcare. Dr. Goodenow said: “I am excited to join Alanis and support the mission to expand the therapeutic options for patients with MDS/AML. I am honored to become a part of team that has developed the jagged-1 antibody to date in collaboration with the Columbia luminaries in this field.”
November 12, 2023 Alanis appoints Cindy Collins to the Board of Directors.
Alanis Therapeutics Inc. (ATI) announced today the appointment of Cynthia (Cindy) Collins to the ATI Board of Directors. Ms. Collins brings a wealth of experience from her extensive managerial and executive experience as the CEO of Editas, Human Longevity, GenVec and Sequoia Pharmaceuticals along with numerous Board positions. Dr. Robert Goodenow, Ph.D., CEO of ATI, said: “We are very pleased to have Cindy as a Board Member since she brings enormous talent and experience as a leader within the industry. Her track record of advancing technologies at the forefront of innovation will be a critical addition to our programs.”
September 10, 2024 SBIR grants $2.0M Phase 2 Award for the Development of Alanis Program.
AvantGen Inc. and Alanis Therapeutics Inc. (ATI) today jointly announced the grant of a $2.0M SBIR award to support the development of ATI’s antagonist antibody directed against jagged-1 for the treatment of MDS/AML. The phase 2 award builds on the collaboration with Columbia University showing that inhibition of notch 1 via binding of the ligand jagged-1, over-expressed by osteoblasts in up to 75% of patients with high-risk MDS/AML, is an oncogenic driver for leukemic stem cells and potential therapeutic target for this malignancy. Dr. Xiaomin Fan, CEO of AvantGen and Founder of both AvantGen and ATI who led the grant process, said: “I am gratified to see the recognition of our efforts and collaboration with Columbia University advance with the support from the SBIR. I am anticipating the treatment of the first MDS/AML patient with our therapeutic antibody to confirm our hypothesis and novel approach.”
November 5, 2024 Alanis Enters Option and License Agreement for AvantGen’s NK Engager and Other Assets.
AvantGen Inc. and Alanis Therapeutics Inc. (ATI) jointly announced the execution of an option and license agreement between the Companies for the development of AvantGen’s recombinant human antibody constructs as therapeutics for the treatment of various malignant and autoimmune diseases. This includes AvantGen’s latest NK engager bi- and tri-specific candidates that can be directed against B cell malignancies as well as autoimmune diseases such as lupus erythematosus. Dr. Xiaomin Fan, Ph.D., CEO and Founder of both AvantGen and ATI, said: “I am excited to have some of our key assets from our antibody engineering platform advance to development with ATI.” Dr. Robert Goodenow, Ph.D., CEO of ATI said: “We are excited to expand our pipeline with the innovative antibodies generated by AvantGen that have the potential to expand therapeutic options for patients with serious unmet clinical need in oncology and autoimmunity.”
October 10, 2025 Alanis Therapeutics Announces Collaboration with Blood Cancer United’s Beat AML Initiative to Advance ATI-D1 as a First-in-Class Therapy for MDS/AML
San Diego, CA — Alanis Therapeutics Inc. (“ATI”), together with Columbia University, will collaborate with The Leukemia & Lymphoma Society—now Blood Cancer United—and its Beat AML team, to explore targeting Notch-1 oncogenic signaling pathway as a novel treatment option for patients with AML.
In this collaboration, patient materials from the Beat AML Master Trial with select molecular subtypes will be assessed for over-expression of components of the Notch-1 signaling pathway that mediates communication between osteoblasts and leukemic stem cells. ATI’s proprietary antibody to Jagged-1—the ligand of Notch-1 will be used in an assay developed by Dr. Stavroula Kousteni, Professor at Columbia University.
Alanis has analyzed dozens of MDS/AML patient samples with diverse genetic mutations. The results consistently demonstrate that whenever the Jagged-1/Notch-1 pathway is activated, ATI-D1, Alanis’s lead therapeutic antibody, effectively inhibits MDS/AML proliferation, induces apoptosis, and promotes stem cell differentiation. ATI-D1 is currently in development as a first-in-class therapy for MDS/AML. The collaboration with Beat AML will allow Alanis to expand the patient sample pool to determine if these findings hold true across broader molecular subtypes.
“It is important to determine whether certain AML molecular subtypes predominate in their over-expression of Notch-1 oncogenic signaling when designing our clinical strategy for ATID1,” said Dr. Bob Goodenow, CEO of Alanis Therapeutics Inc. “Since the pathway—active in the bone marrow of up to 70% of patients—is a contributing factor to minimal residual disease, it is crucial to focus on those phenotypes associated with the poorest outcomes,
as revealed by Beat AML.” For more information, visit https://alanistx.com.
About the Beat AML® Master Clinical Trial
The Beat AML® Master Clinical Trial is the first collaborative precision medicine clinical trial in a blood cancer. Launched by The Leukemia & Lymphoma Society—now Blood Cancer United—in 2016, the trial uses advanced genomic technology to assign patients to the best fit sub-study arm based on their unique genetic characteristics. While initially focused on newly diagnosed AML patients aged 60 or older, the trial has expanded to include relapsed and refractory AML patients.
The Beat AML Master Clinical Trial tests multiple therapies in separate sub-study protocol arms simultaneously that have the power to bring new therapies to AML patients faster. The Master Clinical Trial has already generated strong results, showing superior survival rates and better quality of life when genomic analysis is used to match patients to targeted therapies. Patient samples collected in the Master Clinical Trial Protocol have been used to foster collaborative research projects for understanding the disease and discovery of new therapeutics or diagnostics. For more information, visit www.bloodcancerunited.org/beataml.
PUBLICATIONS

Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, Khiabanian H, Lee A, Murty VV, Friedman R, Brum A, Park D, Galili N, Mukherjee S, Teruya-Feldstein J, Raza A, Rabadan R, Berman E, Kousteni S.
Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts.
Nature. 2014 Feb 13;506(7487):240-4. doi: 10.1038/nature12883. Epub 2014 Jan 15.
Aruna Kode, Ioanna Mosialou, Sanil J. Manavalan, Choza V. Rathinam, Richard A. Friedman, Julie Teruya-Feldstein, Govind Bhagat, Ellin Berman, and Stavroula Kousteni
FoxO1-Dependent Induction of Acute Myeloid Leukemia by Osteoblasts in Mice
Leukemia. 2016 January ; 30(1): 1–13. doi:10.1038/leu.2015.161
Galán-Díez M, Cuesta-Domínguez Á, Kousteni S.
The Bone Marrow Microenvironment in Health and Myeloid Malignancy.
Cold Spring Harb Perspect Med. 2018 Jul 2;8(7):a031328. doi: 10.1101/cshperspect.a031328.
Witkowski MT, Kousteni S, Aifantis I.
Mapping and targeting of the leukemic microenvironment.
J Exp Med. 2020 Feb 3;217(2):e20190589. doi: 10.1084/jem.20190589.
Mosialou, J, Ali, AM, Adams, R, Corper, A, Woods, CM, Fan, X, Raza, A, Kousteni, S.
Therapeutic Anti-Jagged1 Antibody Targeting Osteoblast-Related Myeloid Dysplasia to Overcome Standard of Care Resistance
Blood 2022 140 (Supplement 1) 2902
Bill Liao, Christine Tumanut, Lin Li, Adam Corper, Dilip Challa, Alex Chang, Hydari Begum, Elinaz Farokhi, Catherine Woods & Xiaomin Fan
Identification of novel anti-CD16a antibody clones for the development of effective natural killer cell engagers
mAbs, 2024 16:1, 2381261, DOI: 10.1080/19420862.2024.2381261

